

#Bohr atom model how to
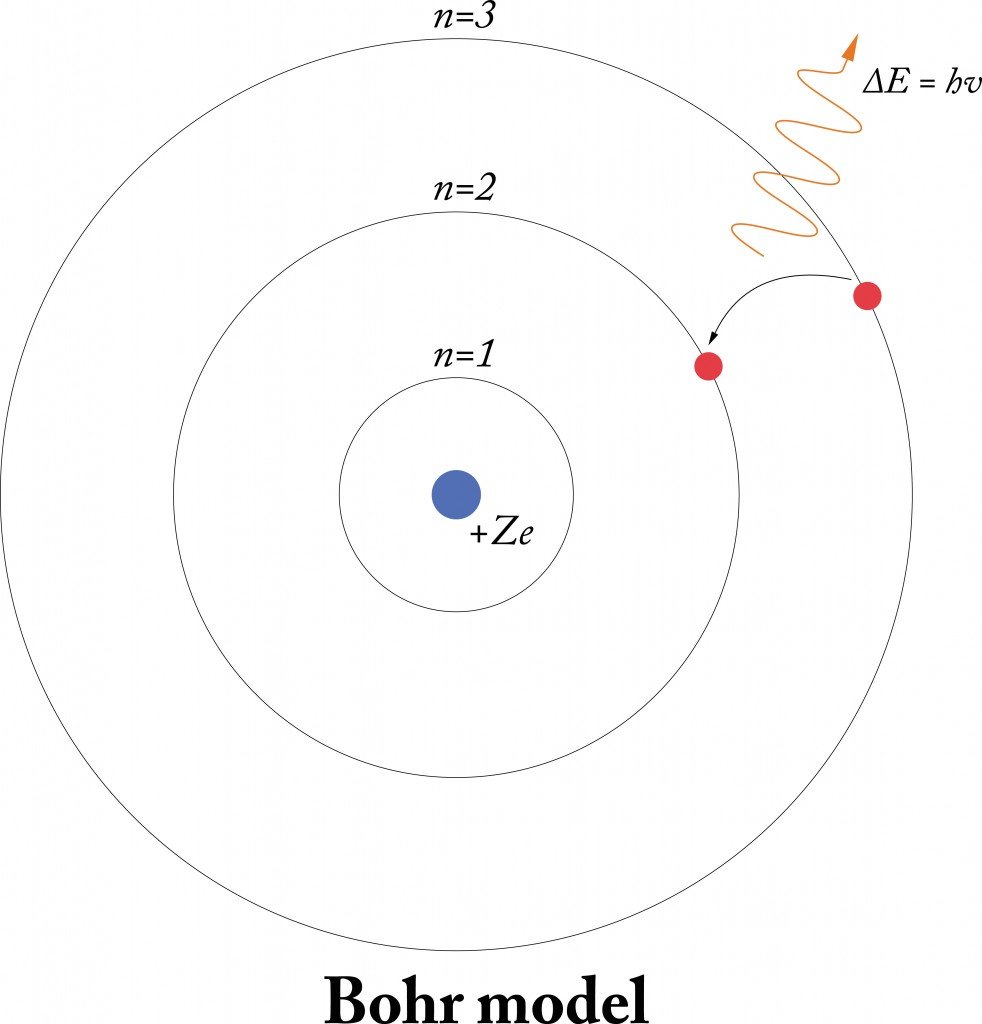
The complete line spectra for the transitions represented in this animation are shown below (note, a hydrogen atom has additional lines related to transitions not shown here). The correct theory of the atom is called quantum mechanics the Bohr Model is an approximation to quantum mechanics that has the virtue of being much simpler. How to Make a 3D Bohr Model of Atoms This is an inexpensive method for making physical Bohr models using pony beads, glass beads, fishing line, wire, and glue. BohrSommerfeld theory is named after Danish physicist Niels Bohr and German physicist Arnold Sommerfeld. As Bohr had noticed, the radius of the n 1 orbit is approximately the same size as an atom. The neon atom, which we will be focusing on. The animation provides data on the energy added and released and the resulting wavelengths of light emitted. The BohrSommerfeld model (also known as the Sommerfeld model or BohrSommerfeld theory) was an extension of the Bohr model to allow elliptical orbits of electrons around an atomic nucleus. In Bohr’s model, radius a n of the orbit n is given by the formula a n h 2 n 2 0 / 2, where 0 is the electric constant. In middle school science, or basic chemistry, the concept of the Bohr atom is introduced, with atomic orbitals. This animation allows you to add different amounts of energy to see how the electron in a hydrogen atom responds, and then manually allow that electron to relax to specific orbitals within the atom (note that in nature electrons relax spontaneously and instantaneously). Bohr’s theory explained that those spectral emission lines were due to the energy released by the relaxation of excited electrons between specific orbitals. Evidence that led to this proposal was the observation of line spectra – distinct bands of light emitted by atoms after they were excited by heat or electricity. In other words, Bohr’s work suggested that electrons do not move freely around the atom, but can only occupy specific energy levels within the atom. In 1913, Neils Bohr built on the work of Max Planck and Albert Einstein and proposed that the movement of electrons within an atom was quantized. In atomic physics, the Bohr model or RutherfordBohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.


 0 kommentar(er)
0 kommentar(er)
